Reveal details of your chemical's molecular structure in solids and liquids
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful tool in structural identification and characterization of pharmaceuticals and other chemicals. NMR spectroscopy is used to unambiguously identify known and novel compounds. NMR is often used to satisfy the regulatory and research requirements of "identity" of a chemical or series of chemicals. NMR can be used to determine how the atoms of a particular molecule are interconnected in either the solid or liquid phase. This is done by analyzing the chemical environment of a selected atomic nucleus. All isotopes that contain an odd number of protons and/or neutrons have an intrinsic nuclear magnetic moment and angular momentum, in other words a nonzero nuclear spin, while all nuclides with even numbers of both have a total spin of zero. The most commonly used nuclei are ¹H (proton) and ¹³C (carbon).Chemical Characterization
NMR spectroscopy is used to unambiguously identify known and novel compounds and much more...
NMR services Triclinic Labs offers:
- NEW cGMP Liquids Analysis
- Chemical structure identification (solids and liquids)
- 1D ¹H and ¹³C NMR spectroscopy with 15N, 19F, 31P, available for routine analyses
- 2D NMR analyses: COSY, NOESY, TOCSY, HMQC, HSQC, HMBC with resonance assignments to provide detailed information on chemical structure and conformation. Rapid interpretation of spectra
- Determining absolute purity
- Primary reference material (PRM) verification
- Verification and execution of USP, EP, JP other compendial NMR methods
-
Characterization of polymorphs, solvates, salts,
cocrystals, and
non-crystalline (amorphous) materials
- Identifying a generic API
- Qualitative and quantitative NMR method development, validation, release testing, method transfer
- Identification and quantification of impurities and contaminants (solids and liquids)
-
Analysis of formulations (deformulation of solids and liquids)
- Identity confirmation of excipients used in pharmaceutical formulations
- Analysis of stereoisomers (solids and liquids)
- Product Failure Analysis
- Confirm the number of molecules in the asymmetric unit (solids)
- Conformational and structural analysis (solids and liquids)
- Chemical exchange analysis (solids and liquids)
- Molecular mobility analysis (solids and liquids)
- Comparability studies of biologics (biosimilarity)
- Patent prosecution and litigation support. Expert (testifying and non-testifying) services, reproduction of prior art. See our Chemical Intellectual Property Expert Services page (click here) for more details
Our Instruments and Capabilities:
| Application AND Technique Description | ||
|---|---|---|
|
By studying the peaks of nuclear magnetic resonance (NMR) spectra, the structure of many compounds
can be determined. It can be a very selective technique, distinguishing among many atoms within a
molecule or collection of molecules of the same type but which differ only in terms of their local
chemical environment. NMR spectroscopy is used to unambiguously identify known and novel compounds.
All isotopes that contain an odd number of protons and/or neutrons have an intrinsic nuclear
magnetic moment and angular momentum, in other words a nonzero nuclear spin, while all nuclides
with even numbers of both have a total spin of zero. The most commonly used nuclei are 1 H and 13 C.
|
||
| Instrument | Models, Probes, Software | Additional Info |
| Bruker | 400MHZ ultrashield, Avance AVII cGMP and non cGMP Liquids, multi-nuclear (1H, 13C, 31P, 19F, 15N, and others with broadband probe) non cGMP Solids, multi-nuclear (13C, 31P, and 19F and others with broadband probe) Topspin 3.2 Software as well as multiple spectral analysis software packages |

In support of The United States Pharmacopoeia (USP) General Chapter <761> Nuclear Magnetic Resonance Spectroscopy and other Compendial chapters (e.g., <1761>), Triclinic Labs offers multi-dimensional cGMP liquids analysis with a broadband probe allowing for a myriad of experiments (1H, 13C, 15N, 19F, 31P, etc.) at variable temperatures (233 - 350K). |
How does NMR work?
When nuclei are placed into a magnetic field, their magnetic moment (spin) becomes aligned with the magnetic field. NMR uses a pulse of radio frequency energy to deflect the nuclei. When the energy is removed, the nuclei relax back to their original state and emit an electromagnetic pulse. A coil inside the NMR receives this RF pulse, and a computer transforms the signal into a spectral graph, which can be interpreted by a spectroscopist. Each nucleus that resides in a unique chemical environment, based upon the chemical structure, translates into a peak in the NMR spectrum. Its peak position (x-axis chemical shift value) and the multiplicity of the peak tells the scientist about its environment. The area of the peak corresponds to the number of nuclei producing the signal so this relationship allows for quantitative analysis.
A combination of 1-dimensional and 2-dimensional NMR experiments are usually necessary for complete confidence in complex chemical structures. The process of discerning structure typically involves:
-
1-dimensional proton ¹H-NMR and Carbon NMR are used to confirm the number of hydrogens and carbons in the molecule, respectively.
-
¹H-¹H Correlation Spectroscopy (COSY) identifies the correlation between hydrogens
- ¹H-¹³C Heteronuclear Single Quantum Coherence Spectroscopy (HSQC) identifies which hydrogens are attached to which carbon atoms.
- ¹H-¹³C Heteronuclear Multiple Bond Correlation Spectroscopy (HMBC) shows the correlations between protons and carbons with multiple bonds
-
Other nuclei may be explored (e.g. 19F, 15N, 31P)

Pharmaceutical Applications
Uses of NMR in Current Guidance for Pharmaceutical Quality/CMC
Under current regulations in the United States, use of a human drug product not previously authorized
for marketing in the United States requires the submission of an IND to the Agency. FDA's regulations
at 21 CFR 312.22 and 312.23, respectively, contain the general principles underlying the IND submission
and the general requirements for content and format. Section 312.23(a)(7)(i) requires that an IND for
each phase of investigation include sufficient Chemistry Manufacturing and Controls (CMC) information
to ensure the proper identity, strength or potency, quality, and purity of the drug substance and drug
product. The FDA or other regulatory agencies usually require full structural characterization by NMR.
This data provides crucial evidence of compound identity.
Other CMC Services we offer:
- Chemical name
- Structure figure
- Molecular formula
- Molecular weight
- Elemental analysis (CHN)
- UV/VIS spectroscopy
- IR spectroscopy
- Raman spectroscopy
- 1H NMR spectroscopy
- 13C NMR spectroscopy
- Mass spectrometry
- Optical, Specific rotation
- Phase identification by XRPD
- Ion Chromatography
- Single crystal X-ray
See a complete description of our CMC services. Click here.
Quantitative NMR
Quantitative NMR, or qNMR, is used for determination of concentration and purity of small molecules, for example. The qNMR method only requires that (a) the sample dissolves completely in a (normally fully deuterated) solvent, and (b) it contains NMR-active nuclides.
There are two broad categories of qNMR experiments: those used to determine the analyte concentration, and those used to determine a compound's purity.
Quantitative Nuclear Magnetic Resonance (qNMR) Spectroscopy Services
- qNMR can provide fast and low-cost quality control (QC) tests such as identification, purity and potency tests, without the corresponding reference standard materials.
- qNMR is also useful in investigation of unknowns and product failure.
What is qNMR?
NMR spectroscopy analyzes molecules at the atomic level, detecting protons or carbons, making NMR one of the most universal techniques. qNMR takes advantage of the fact that the peak area (integration) in NMR spectroscopy is directly proportional to the concentration of the specific component in solution. This feature allows for determination of relative concentration of each component in a mixture. For absolute quantitation, a certified reference material (CRM) is utilized either as an internal or external standard. The CRM is not chemically related to any of the components in question and therefore the same CRM can be used for quantitation of wide variety of test articles.Why qNMR?
NMR spectroscopy is one of the most universal techniques used in identification of chemical components. qNMR is a relatively simple but versatile technique that can perform the identification testing and simultaneously determine the concentration of one or more components in a test article without having the requirement of certified reference standard materials of each of the corresponding compounds. qNMR can be used for purity assay, potency determinations, investigation of unknowns, investigation of product failures, and as a part of quality control (QC) tests.
qNMR and Regulatory Requirements
United States Pharmacopeia (USP) chapter <761> Nuclear Magnetic Resonance Spectroscopy describes qNMR under Qualitative Applications. For absolute quantitation, a CRM is used as an internal or an external standard. The internal standard is co-dissolved in the solution with test articles. The external standard solution is placed in an insert within the NMR tube that contains the test solution. The CRM is not chemically related to any of the components in question. This simplifies the GMP validation procedure since a limited number of CRM in a small number of solvents can be validated for linearity, range, accuracy, precision, quantitation limit and robustness. For each new test article, only specificity needs to be validated as long as the same solvent system and experimental parameters are used.
Pros and Cons of qNMR
Pros of qNMR:Cons of qNMR:
- qNMR is a primary method where the signal intensity depends directly on the number of protons and concentration.
- Quick and simple analysis of test articles to identify and quantify components. Quantitative information such as purity of drug substances, potency of drug products can be obtained by qNMR.
- Unlike chromatographic methods, reference standard materials of the corresponding compounds are not required. This is especially advantageous during drug product development when reference standards of the active ingredients and impurities are not available.
- Unlike HPLC-UV methods, there is no response factor calculation required for active ingredients and impurities.
- Unlike HPLC methods, water or volatile content analysis (by KF or TGA) are not required for the purity determination.
- A number of CRM are commercially available.
- Applied to components with known chemical structures.
- The CRM selected must have a specificity against the NMR peaks related to the test sample.
- The test article must have detectable nuclei (such as proton or carbon).
- For mixture analysis, all components of interest must be soluble in a deuterated solvent or solvents.
Applications of qNMR
- Identification and purity testing of pure components such as active pharmaceutical ingredients (APIs) and excipients.
- Identification and potency testing of drug product intermediates during product development and stability testing.
- Identification and potency testing, as a part of a release and stability study, of drug product intermediates and final products such as tablets, capsules, solutions, suspensions, granules, and powders.
- Concentration determination of each component in mixtures. The mixtures can be solids, solutions or suspensions.
- Investigation of product failure
- Food sciences

Mini Case Study:
qNMR Potency Testing of Generic Excedrin Tablets
Three different generic brands claiming equivalence to Excedrin Migraine Tablets were dissolved in DMSO-d6 with maleic acid as an internal standard. The label claims were evaluated and are provided in the table below. None of the generics we tested matched the label claims.
| Components | Dosage Label Claim (mg/tablet) |
Actual Dosage
Product 1 |
Actual Dosage
Product 2 |
Actual Dosage
Product 3 |
| Acetaminophen | 250 | 98% | 83% | 110% |
| Aspirin | 250 | 101% | 98% | 66% |
| Caffeine | 65 | 115% | 115% | 107% |
| Comments | Contains Salicylic acid (See Figure Below) |
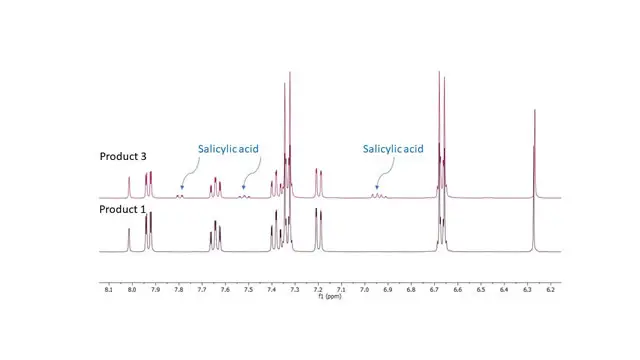
Figure 1. Salicylic acid is an active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prodrug to salicylic acid, it is probably best known for its use as a key ingredient in topical anti-acne products.
